| ■Osumi Group embryo |
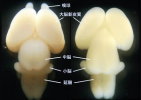
■Osumi Group postnatal & adult brain |
■Wakamatsu Group Avian neural crest |
|
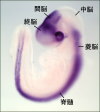
In order to obtain complex functions in the brain, certain numbers of neurons of various kinds should be generated and localize in precise positions, and project their axons to thier appropriate targets. These processes are largely controlled by genetic programs. Our aim is to understand the regulatory mechanisms of neuronal development at cellular and molecular levels, i.e., proliferation, differentiation, and migration of neurons, which are required for proper formation of neuronal networks. We are especially interested in the following topics  E12.0 rat embryo (neurofilament staining) E12.0 rat embryo (neurofilament staining)
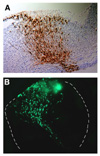
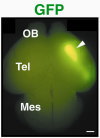
Various types of neurons are born in the hindbrain, the primordium of the brain stem, along the anterior-posterior and dorsal-ventral axes. We have analyzed molecular mechanisms that control specification of these neurons in the hindbrain. Our results have revealed that various kinds of neurons are specified at the precursor stage by combinatorial expressions of transcriptional factors that are differentially expressed according to their positions in the developing brain 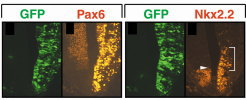 【FIg.3】Inhibition of Pax6 activity by dominat negative form of Pax6 in the hindbrain The olfactory bulb and the pyriform cortex are centers for olfaction and belong to the limbic system. The olfactory bulb is initially formed as a protrusion of the anterior end of the telencephalon, while the pyriform cortex develops around the area that corresponds to the boundary of the dorsal and the ventral telencephalon. By cell tracing, cell transplantation, and molecular analyses, we have identified that both the olfactory bulb and the pyriform cortex are mosaic structures containing cells originating from different regions of the telencephalon. We also found that the positioning of the olfactory bulb is intrinsic to the telencephalon and dependent on its initial patterning by a transcriptional factor, Pax6 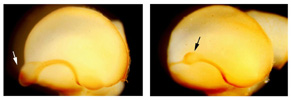 【Fig.4】Abnormal positioning of the olfactory bulb in the Pax6 mutant
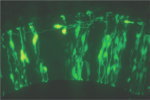
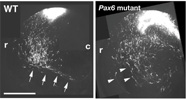 【Fig.6】Abnormal migration of neurons derived from dorsal area in the Pax6 mutant. The neural tube is a primordial structure of the central nervous system and made up with a sheet of pseudostratified epithelial cells called as neuroepithelium. Neuroepithelial cells include neural stem cells that self-renew and produce various kinds of neurons and glial cells. They are highly polarized with long apical and basal processes. Interestingly, nuclei of the neuroepithelial cells move up and down during the cell cycle (i.e., interkinetic nuclear movement; those in DNA synthesis are positioned in the basal side, and those during cell division are in the apical side. The cell fate of daughter cells can be categorized into three classes: two neuroepithelial cells, two neurons, or one neuroepithelial cell and one neuron. The former two classes are categorized as symmetrical division, while the third is considered as asymmetrical division. Factors involved in the cell fate determination are localized asymmetrically prior to the asymmetrical cell division. Daughter cells fated to neurons move toward the basal side (radial migration), and in some cases migrate tangentially to their final destinations. We monitor these fascinating behaviors of neural progenitor cells and neurons by time-lapse imaging of the developing brain tissues
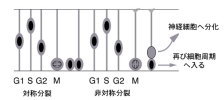 【Fig.8】Interkinetic nuclear movement and cell cycle  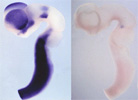 【Fig.9】 Identification of Pax6 target, Fabp7, by microarray analysis The expression of Fabp7 is dounregulated in the Pax6homozugous mutant rat. We are also developing various techniques for above studies. For example, we have established a novel technique directly transferring exogenous genes into developing tissues by combining whole embryo culture method and electroporation both in mammalian and avian embryos. These techniques allowed us an easy and highly-efficient transfection of genes to target regions and stages of developing embryos, which surpasses other methods like making transgenic mice and transfection with virus vectors. To examine the effects of gene transfer in more developed stages of mammalian embryos, when architectures of the brain become more advanced, we have developed an ultrasound-guided in utero electroporation method
|
|
|
|
| ▲page top▲ |
|