| @@”Osumi group @@ @embryo |
@@”Osumi group postnatal & adult brain |
@”Wakamatsu Group @Avian neural crest |
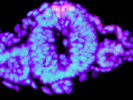
Regulatory Mechanisms for Neural Crest Formation and Differentiation Neural crest is formed at the border of neural and non-neural ectoderm at early stages of vertebrate embryonic development (Fig. 1), and subsequently undergo epithelial-mesenchymal transition (EMT). Neural crest cells eventually give rise to a wide variety of cell types including neurons and glial cells in the peripheral nervous system, pigment cells in the skin (melanocytes), some endocrine cells, smooth muscle cells, and cranio-facial connective tissues and skeletal tissues. Thus, neural crest is literally "the forth germ layer" specific for vertebrates.
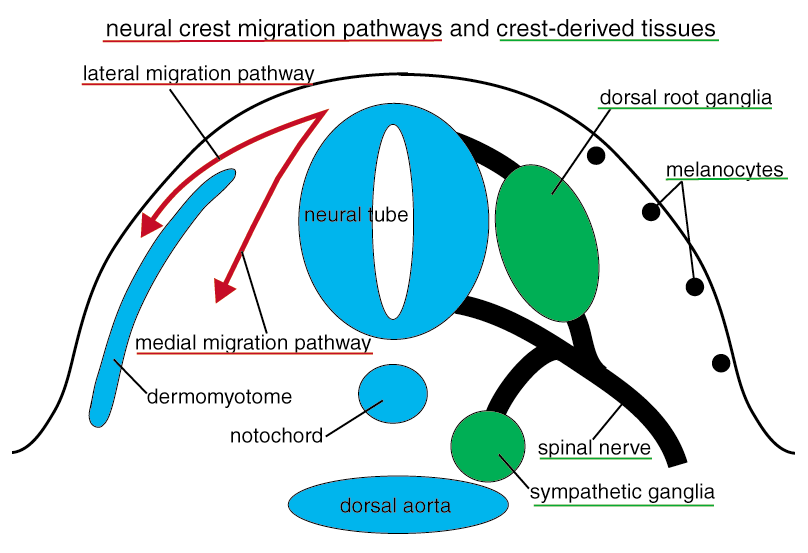
As these crest-derived cell types must colonize distinct locations in the body, neural crest cells migrate extensively during development. For example, neurons and glial cells in the enteric nervous system, enteric crest cells generated at the rostral part of the embryo must migrate and distribute entire gut tube. The neural crest cells forming peripheral nervous system such as dorsal root ganglia and crest-derived melanocyte precursors use distinct migratory pathways specific for their cell types. Our laboratory challenges to understand the mechanisms of these processes including neural crest formation, migration, and differentiation, at cellular and molecular levels.
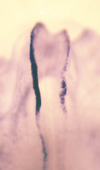
As mentioned above, neural crest is formed at the border of neural and non-neural ectoderm (see a review, Sakai & Wakamatsu, 2005). It has been shown that the interaction between these tissues are important for the neural crest induction, and BMP signal is the prim-suspect of inducing factor from the non-neural ectoderm. We have shown that BMP signal is essential for the induction of the neural crest in avian embryos (Fig. 3, Endo et al., 2002), and that Deltex-mediated Notch signal regulate BMP4 expression in the non-neural ectoderm (Endo et al., 2002 & 2003). BMP signal subsequently induces expression of neural crest-specific transcription factors, such as Snail2 (Endo et al., 2002, Sakai et al., 2005), and Sox9 (Sakai et al., 2006). In addition to the BMP signal, we also showed the importance of cAMP-dependent kinase (PKA) signaling in neural crest induction (Sakai et al., 2006). For example, PKA phsophorylates Sox9, and this phsophorylation is required for Sox9 to promote EMT of neural crest cells (Sakai et al., 2006). PKA signal also regulates the activity of Snail2 and Sox10, and Sox10 activates the expression of type2 Collagen in migrating neural crest cells (Suzuki et al., 2006). Unlike Sox9 and Sox10, Sox2 is expressed in the neural ectoderm to inhibit neural crest induction and EMT (Wakamatsu et al., 2004a).
Among the neural crest-specific transcription factors, Snail2 (formerly known as Slug) has been known to promote EMT. We have identified the promoter region of chick Snail2 gene, which is sufficient for the expression of Snail2 in neural crest cells (Sakai et al., 2005). We showed that Smad1, a mediator of BMP signal, directly binds to the promoter and activates the transcription. Furthermore, Sox9 and Snail2 form a complex and bind to the promoter sequence to activate the promoter, under the influence of PKA signal (Sakai et al., 2006). We are currently working on identifying more factors to regulate neural crest formation and EMT in the downstream of PKA signal. The mechanisms for neural crest migration have been studied for long time. The neural crest cells preferentially migrate on extra-cellular matrix-rich environment, by using Integrin. Medially-migrating neural crest cells (Fig. 2) avoid caudal halves of developing somites, resulting in segmental formation of the dorsal root ganglia (Fig. 4). This migration pattern is mostly established by the repulsive factors (e. g. Ephrins and F-spondin) expressed in the caudal somites. In contrast, melanocyte precursors choose lateral pathway (Fig. 2), possibly by (an) attractant(s) in this region. This has been shown by the fact that medially-transplanted melanocyte precursors migrate backwards to enter the lateral pathway (Wakamatsu et al., 1998). Neurogenic neural crest cells accidentally enter the lateral pathway will be removed by selective cell death (Wakamatsu et al., 1998).
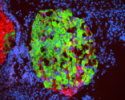
Some neural crest-derived cells have multipotency to give rise to several cell types (Fig. 2). We have previously shown that Notch signaling is involved in the inhibition of neuronal differentiation of crest- derived PNS cells, and that Notch signaling is modulated by the asymmetric inheritance of a fate determinant, Numb, during mitosis of crest-derive precursor cells (Wakamatsu et al., 2000). This mechanism is important to generate neurons and glial cells in the equivalent environment of the developing ganglia (Fig. 5). We also showed that Sox2 inhibits neuronal differentiation in the downstream of Notch signaling (Wakamatsu et al., 2004a). We have also identified other early lineage-specific marker molecules, such as Mitf for melanocyte precursors (Wakamatsu et al., 1998), and Seraf for Schwann cell precursors (Wakamatsu et al. 2004b).
Neural epithelium cells (NE cells) behave as a stem cells to generate neurons and glial cells in the central nervous system. It has been known that Notch signaling inhibits neuronal differentiation of NE cells. We have reported that Numb is asymmetrically localized in mitotic NE cells, and inherited unevenly by the daughter cells (Fig. 6; Wakamatsu et al., 1999). We have recently identified Transitin, an intermediate filament protein, as an anchor for Numb to localize asymmetrically in the mitotic NE cells (Wakamatsu et al., Development in press). We are currently working on how the asymmetric localization and uneven inheritance of Numb-Transitin complex is mediated.
|
||||||||||||
|
|
|
| £pase top£ |
|